Hypha Discovery: Experts in Metabolite Synthesis
Purification
Structure elucidation
Natural Products
Supporting pharmaceutical and agrochemical companies globally

Your trusted partner in pharmaceutical and agrochemical R&D
What we do
Hypha Discovery is a specialist CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and late-stage derivatives of drugs and agrochemicals in discovery and development.
We are experts in the scalable synthesis, purification and identification of drug metabolites and oxidised derivatives of lead compounds, and also possess a wealth of experience in the production, purification and structure elucidation of natural products.
We also use our purification expertise to isolate and identify manufacturing impurities and degradation products.
NMR Spectroscopy and Structure Elucidation
NMR Spectroscopy and Structure Elucidation
• Quantitative NMR
• Interpretation and documentation of spectra
Solutions for metabolite synthesis
Hypha Discovery offer a comprehensive suite of techniques for provision of even the most difficult-to-synthesise metabolite, comprising chemical synthesis, recombinant enzymes such as PolyCYPs, microbial biotransformation, mammalian liver fractions as well as the purification of metabolites from biological matrices. We are also experts in the structure elucidation of metabolites by NMR spectroscopy.
Chemical Synthesis
Late-stage chemical glucuronidation
Mammalian biotransformation
Panels of liver S9s / microsomes
Microbial biotransformation
Proven panels of bacteria and fungi
Purification & structure elucidation
COAs, acquisition / interpretation of NMR data
Recombinant enzymes
PolyCYPs, hrCYPs, AOX, FMOs
PolyCYPs kits
PolyCYPs+ screening kits comprise 18 PolyCYP isoforms together with the addition of other phase 1 enzymes. Human aldehyde oxidase (AOX1) and the main human hepatic flavin-containing monooxygenase (FMO3) are included in the kit, with the other human FMO isoforms, available separately at Hypha.
Inclusion of multiple PolyCYPs isoforms broadens coverage of reactions, and which have been proven to produce human and other mammalian CYP-derived metabolites including multi-step metabolites and metabolites of low turnover drugs.

Simple, Effective, Scalable
Produce CYP, AOX & FMO metabolites
Generate multiple hydroxylated and other oxidised derivatives of lead compounds in parallel
About Us
Delivering a first class service to our clients worldwide
Expertise
Hypha Discovery is your trusted global partner. We are go-to experts for metabolite solutions and have worked with many of the top pharma and agrochemical companies worldwide to deliver projects.
Flexibility
We will work with you to design a custom solution that meets your needs and which can be adapted in response to project milestones.
Service
We pride ourselves on our client focused approach. Our scientists will work with you to personally deliver your project.
We look forward to engaging Hypha in the future because their team is professional, and their services complement our internal synthetic efforts for delivering new molecules.
Hypha Discovery News
Hypha Discovery has a great opportunity for a lab-based chemist with purification experience to join our team in Oxfordshire. Join us and learn from the best!
Our Q4 2023 newsletter looks at Hypha’s new late-stage oxidation and deoxyfluorination techniques for blocking metabolic soft spots.
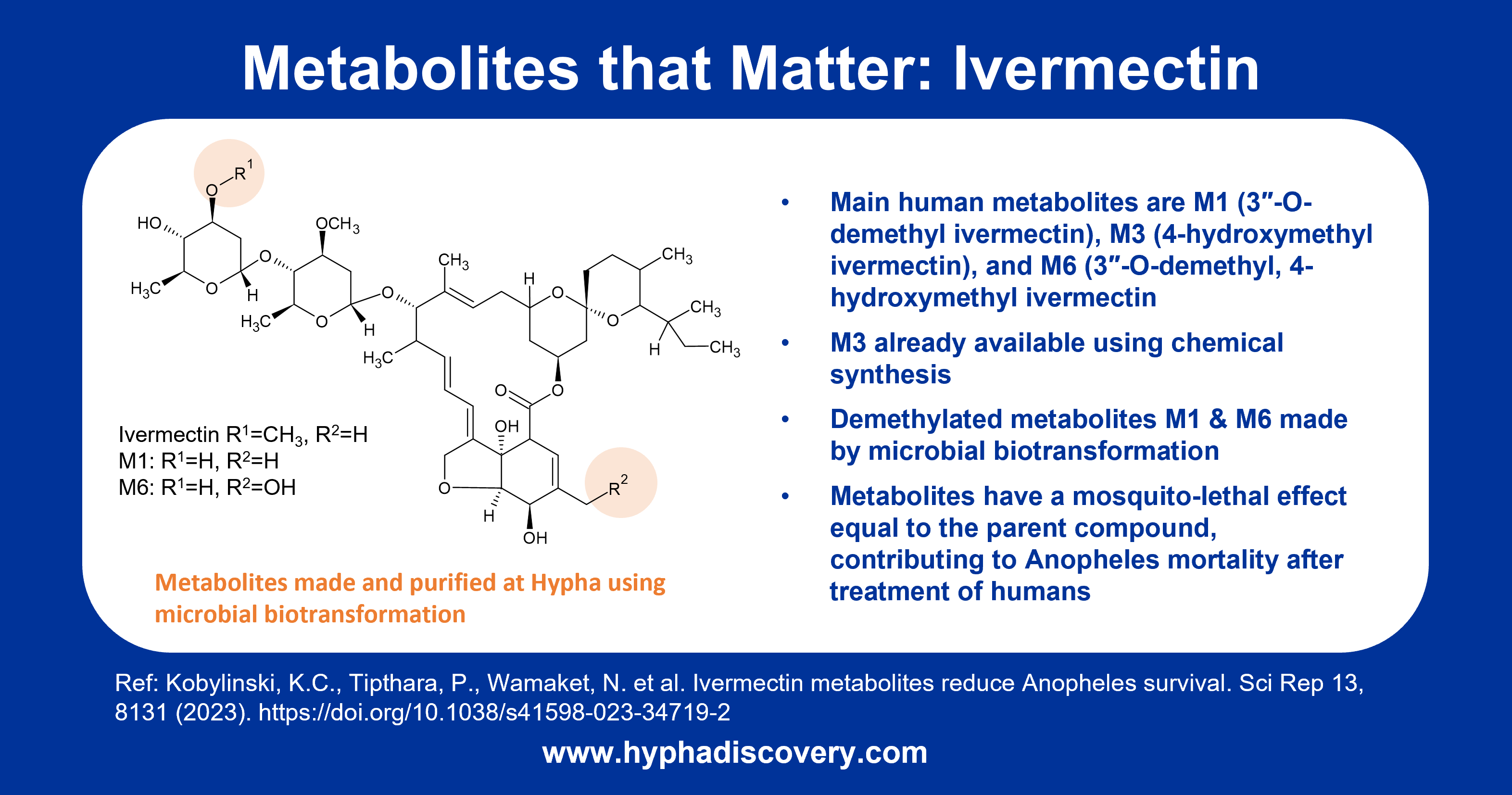
Major O-demethylated human metabolites of ivermectin that reduce Anopheles survival were made by Hypha’s microbial biotransformation system, and are the focus feature of our Q3 2023 newsletter.
Blog
Check out our “Metabolite Tales” blog articles on interesting aspects around drug metabolism and medicinal chemistry.
Brochures
Access brochures on drug metabolite synthesis, Hypha’s one-stop metabolite shop, the PolyCYPs platform and PolarExplorer.
Stay up to date with the latest news from Hypha Discovery
Sign up for our quarterly newsletters and monthly "Metabolite Tales" blog
Ready to begin? Our scientists are available to talk through your requirements
Hypha Discovery is a UK-based CRO supporting pharmaceutical and agrochemical companies worldwide through the production of metabolites and new derivatives of drugs and agrochemicals in discovery and development.
Resources
Cookie Policy | Privacy Policy | Website Terms and Conditions
© Hypha Discovery 2021. All Rights Reserved. Website by Fifteen.co.uk